Internationally Recognized Research in Cell and Tissue Engineering
MTM Lab strives for transformative impact by creating engineered tissues for applications in drug development, disease modeling, and regenerative medicine (cell-based therapies).
Our mission is to improve people's lives by helping to create tools and better treatments to combat adverse drug effects and chronic organ diseases.
Director: Salman R. Khetani, PhD

UNLOCK YOUR POTENTIAL
Join our vibrant team of researchers and gain access to cutting-edge cell and tissue engineering research, advanced academic opportunities, and job placements in industry and academia.
What's new & exciting

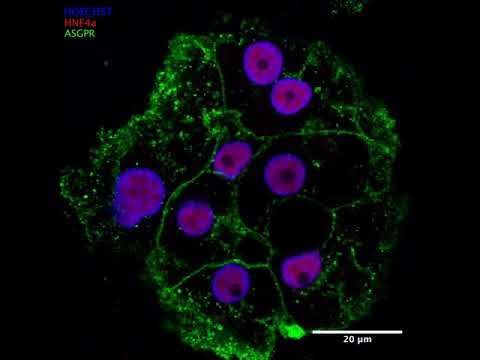
List of Services
-
Long-term HBV infection of engineered cultures of induced pluripotent stem cell-derived hepatocytes
Yuan Y, Bodke VV, Lin C, Gao S, Rehman J, Li J, Khetani SR. Long-term HBV infection of engineered cultures of induced pluripotent stem cell-derived hepatocytes. Hepatol Commun. 2024 Jul 31;8(8):e0506. doi: 10.1097/HC9.0000000000000506. PMID: 39082962.
-
Engineered cocultures of iPSC-derived atrial cardiomyocytes and atrial fibroblasts for modeling atrial fibrillation
Grace E. Brown et al. ,Engineered cocultures of iPSC-derived atrial cardiomyocytes and atrial fibroblasts for modeling atrial fibrillation. Sci. Adv. 10, eadg1222 (2024). DOI: 10.1126/sciadv.adg1222
-
Decellularized Liver Nanofibers Enhance and Stabilize the Long-Term Functions of Primary Human Hepatocytes In Vitro
Liu JS, Madruga LYC, Yuan Y, Kipper MJ, Khetani SR. Decellularized Liver Nanofibers Enhance and Stabilize the Long-Term Functions of Primary Human Hepatocytes In Vitro. Adv Healthc Mater. 2023 Jul;12(19):e2202302.
-
A human iPSC-derived hepatocyte screen identifies compounds that inhibit production of Apolipoprotein B.
Liu JT, Doueiry C, Jiang YL, Blaszkiewicz J, Lamprecht MP, Heslop JA, Peterson YK, Carten JD, Traktman P, Yuan Y, Khetani SR, Twal WO, Duncan SA. A human iPSC-derived hepatocyte screen identifies compounds that inhibit production of Apolipoprotein B. Commun Biol. 2023 Apr 24;6(1):452.
-
Engineered Platforms for Maturing Pluripotent Stem Cell-Derived Liver Cells for Disease Modeling
Yuan Y, Cotton K, Samarasekera D, Khetani SR. Engineered Platforms for Maturing Pluripotent Stem Cell-Derived Liver Cells for Disease Modeling. Cell Mol Gastroenterol Hepatol. 2023;15(5):1147-1160.
-
Atrial Fibrillation ModelList Item 1
Mature Patient Stem Cell-Derived Atrial Cardiomyocytes Unmask Ion Channel and Metabolic Defects in an NPPA Atrial Fibrillation Model. JCI Insight (2022)
-
See All MTM Lab Publications >>